The Universe is now 1 minute old, and all the anti-matter has been destroyed by
annihilation with matter. The leftover matter is in the form of electrons, protons and
neutrons. As the temperature continues to drop, protons and neutrons can undergo fusion
to form heavier atomic nuclei. This process is called nucleosynthesis.

Its harder and harder to make nuclei with higher masses. So the most common substance
in the Universe is hydrogen (one proton), followed by helium, lithium, beryllium and
boron (the first elements on the periodic table). Isotopes are formed, such as deuterium
and tritium, but these elements are unstable and decay into free protons and neutrons.
Note that this above diagram refers to the density parameter, Omega, of baryons, which is
close to 0.1. However, much of the Universe is in the form of dark matter (see later
lecture).
A key point is that the ratio of hydrogen to helium is extremely sensitive to the density of
matter in the Universe (the parameter that determines if the Universe is open, flat or
closed). The higher the density, the more helium produced during the nucleosynthesis era.
The current measurements indicate that 75% of the mass of the Universe is in the form of
hydrogen, 24% in the form of helium and the remaining 1% in the rest of the periodic
table (note that your body is made mostly of these `trace' elements). Note that since
helium is 4 times the mass of hydrogen, the number of hydrogen atoms is 90% and the
number of helium atoms is 9% of the total number of atoms in the Universe.

There are over 100 naturally occurring elements in the Universe and classification makes
up the periodic table. The very lightest elements are made in the early Universe. The
elements between boron and iron (atomic number 26) are made in the cores of stars by
thermonuclear fusion, the power source for all stars.
The fusion process produces energy, which keeps the temperature of a stellar core high to
keep the reaction rates high. The fusing of new elements is balanced by the destruction of
nuclei by high energy gamma-rays. Gamma-rays in a stellar core are capable of disrupting
nuclei, emitting free protons and neutrons. If the reaction rates are high, then a net flux of
energy is produced.
Fusion of elements with atomic numbers (the number of protons) greater than 26 uses up
more energy than is produced by the reaction. Thus, elements heavier than iron cannot be
fuel sources in stars. And, likewise, elements heavier than iron are not produced in stars,
so what is their origin?.

The construction of elements heavier than involves nucleosynthesis by neutron capture. A
nuclei can capture or fuse with a neutron because the neutron is electrically neutral and,
therefore, not repulsed like the proton. In everyday life, free neutrons are rare because
they have short half-life's before they radioactively decay. Each neutron capture produces
an isotope, some are stable, some are unstable. Unstable isotopes will decay by emitting a
positron and a neutrino to make a new element.

Neutron capture can happen by two methods, the s and r-processes, where s and r stand
for slow and rapid. The s-process happens in the inert carbon core of a star, the slow
capture of neutrons. The s-process works as long as the decay time for unstable isotopes is
longer than the capture time. Up to the element bismuth (atomic number 83), the s-process
works, but above this point the more massive nuclei that can be built from bismuth are
unstable.
The second process, the r-process, is what is used to produce very heavy, neutron rich
nuclei. Here the capture of neutrons happens in such a dense environment that the
unstable isotopes do not have time to decay. The high density of neutrons needed is only
found during a supernova explosion and, thus, all the heavy elements in the Universe
(radium, uranium and plutonium) are produced this way. The supernova explosion also
has the side benefit of propelling the new created elements into space to seed molecular
clouds which will form new stars and solar systems.
Ionization:
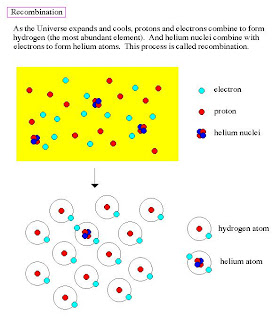
The last stage in matter production is when the Universe cools sufficiently for electrons to
combine with the proton/neutron nuclei and form atoms. Constant impacts by photons
knock electrons off of atoms which is called ionization. Lower temperatures mean
photons with less energy and fewer collisions. Thus, atoms become stable at about 15
minutes after the Big Bang.


No comments:
Post a Comment